We are developing new oral investigational medicines which are designed to provide sustained, fat-selective, muscle-preserving weight loss for patients with MASH, obesity, and associated cardiometabolic diseases.
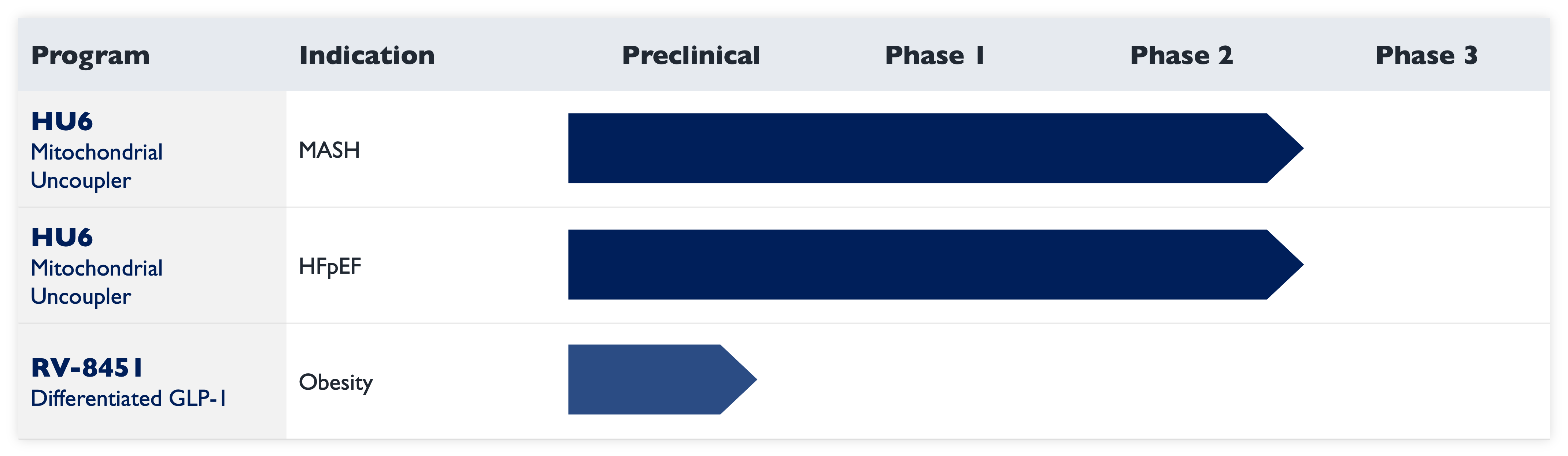
Our lead candidate HU6 is a best-in-class mitochondrial uncoupler that has been evaluated in three Phase 2 clinical trials, all of which met their primary endpoints. HU6 has demonstrated a favorable efficacy, safety, and tolerability profile in these Phase 2 trials in MASLD (metabolic dysfunction-associated steatotic liver disease), MASH (metabolic dysfunction associated steatohepatitis), and HFpEF (heart failure with preserved ejection fraction). In clinical trials, HU6 treatment has resulted in robust liver-centric effects, fat-selective, muscle-preserving weight loss, and improvements in cardiovascular risk markers.
HU6 results have been published in The Lancet Gastroenterology & Hepatology for MASLD, presented in a late-breaker oral presentation at AASLD for MASH, and published in JAMA Cardiology for HFpEF. Rivus plans to conduct an additional Phase 2 trial in MASH, a disease driven by obesity and a growing public health issue for which new therapeutic options are urgently needed.
Rivus’ pipeline of oral therapies includes RV-8451, a differentiated oral GLP-1 that is the first muscle-preserving oral GLP-1 non-peptide agonist. RV-8451 is in preclinical development for the treatment of obesity.
RIVUS’ PIPELINE OF ORAL INVESTIGATIONAL MEDICINES
MASH – Metabolic dysfunction-associated steatohepatitis
MASLD – Metabolic dysfunction-associated steatotic liver disease
HFpEF – Heart failure with preserved ejection fraction
ANT – Adenine nucleotide translocase
GLP-1 – Glucagon-like peptide-1 (receptor)
Contact Us
For general inquiries please e-mail us at info@rivuspharma.com.
