Accelerating to better health
Rivus Pharmaceuticals, Inc. is dedicated to improving cardiometabolic health by advancing a new class of investigational medicines called controlled metabolic accelerators (CMAs). These oral small molecules are designed to reduce excess fat and treat a broad range of cardiometabolic diseases by safely leveraging the natural process of mitochondrial uncoupling. Activating this metabolic process results in fat-specific weight loss, preservation of muscle mass, reduction of liver and visceral fat, improved glycemic control and reductions in oxidative stress and inflammation. Rivus’ lead CMA, HU6, is in development to treat obesity and associated cardiometabolic diseases, including heart failure with preserved ejection fraction (HFpEF), metabolic dysfunction-associated steatotic liver disease (MASLD) / metabolic dysfunction-associated steatohepatitis (MASH) and Type 2 diabetes. Rivus’ discovery platform which combines an uncoupling AI/ML classifier model trained on proprietary datasets has yielded additional CMA candidates for preclinical development in therapeutic areas with significant need.

Rivus Pharmaceuticals, Inc. is dedicated to improving cardiometabolic health by advancing a new class of investigational medicines called controlled metabolic accelerators (CMAs). These oral small molecules are designed to reduce excess fat and treat a broad range of cardiometabolic diseases by safely leveraging the natural process of mitochondrial uncoupling. Activating this metabolic process results in fat-specific weight loss, preservation of muscle mass, reduction of liver and visceral fat, improved glycemic control and reductions in oxidative stress and inflammation. Rivus’ lead CMA, HU6, is in development to treat obesity and associated cardiometabolic diseases, including heart failure with preserved ejection fraction (HFpEF), metabolic dysfunction-associated steatotic liver disease (MASLD) / metabolic dysfunction-associated steatohepatitis (MASH) and Type 2 diabetes. Rivus’ discovery platform which combines an uncoupling AI/ML classifier model trained on proprietary datasets has yielded additional CMA candidates for preclinical development in therapeutic areas with significant need.
OUR SCIENCE
Addressing a primary driver of cardiometabolic disease: obesity
Nearly half of American adults are living with cardiometabolic diseases. While numerous factors contribute to these diseases, a common thread driving pathology is obesity, the result of excess fat in the body. Associated with an elevated risk of heart failure, type 2 diabetes, dyslipidemia, MASLD/MASH and other indications, obesity presents an opportunity to target a significant risk factor for cardiometabolic disease. Newer therapies support weight loss but may also reduce lean muscle mass, which can lead to weight regain and increased cardiovascular risk.

Harnessing a natural metabolic process for fat selective weight loss
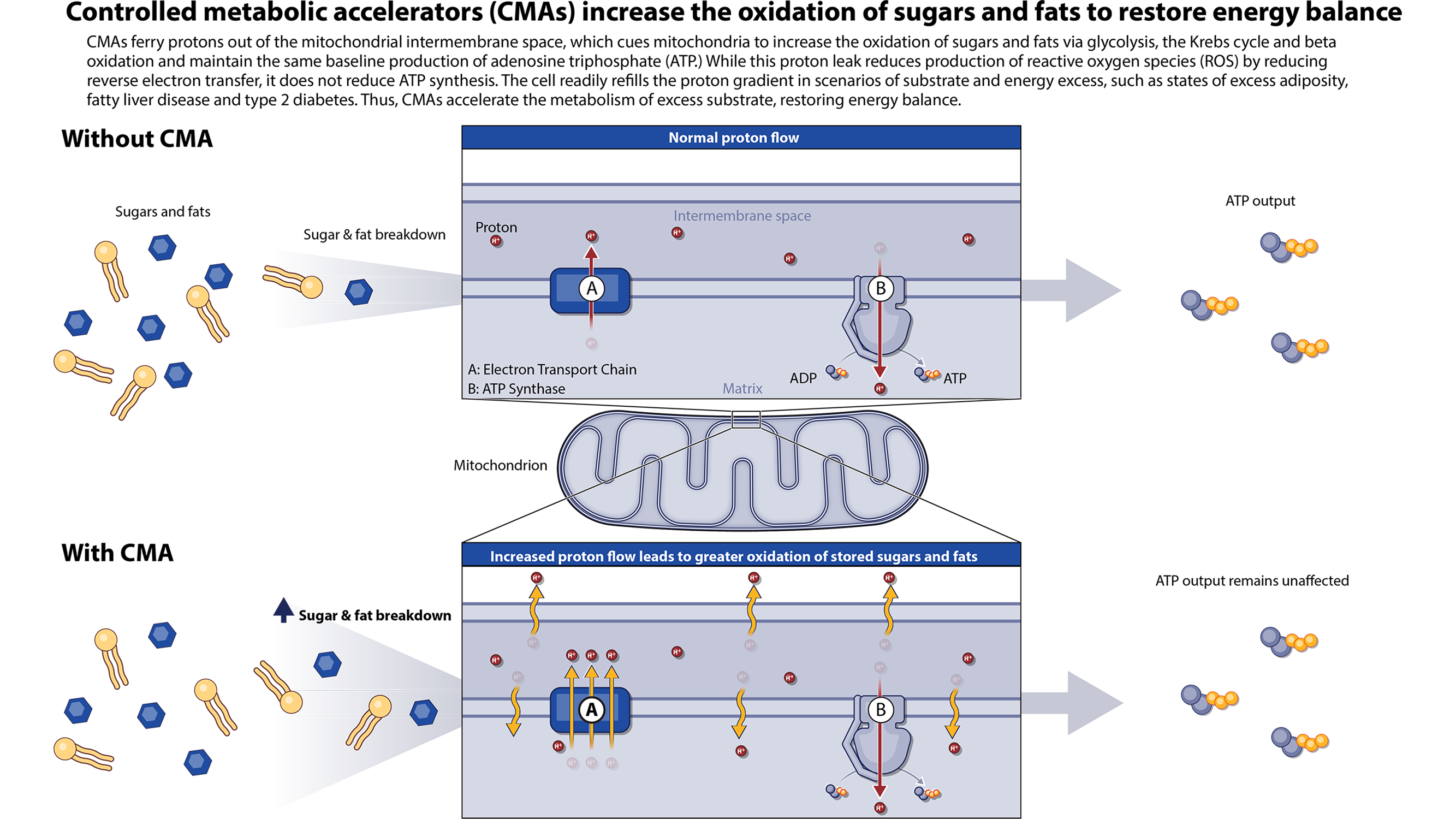
Rivus’ Controlled Metabolic Accelerators (CMAs) are oral small molecules which are designed to reduce excess fat and treat a broad range of cardiometabolic diseases by safely leveraging the natural process of mitochondrial uncoupling. Mitochondrial uncoupling accounts for approximately 20-40% of caloric consumption and significantly contributes to basal metabolic rate. Rivus’ CMAs provide a novel, measured approach to activating this process, reducing fat throughout the body while preserving skeletal muscle mass.
Harnessing a natural metabolic process for fat selective weight loss
Rivus’ Controlled Metabolic Accelerators (CMAs) are oral small molecules which are designed to reduce excess fat and treat a broad range of cardiometabolic diseases by safely leveraging the natural process of mitochondrial uncoupling. Mitochondrial uncoupling accounts for approximately 20-40% of caloric consumption and significantly contributes to basal metabolic rate. Rivus’ CMAs provide a novel, measured approach to activating this process, reducing fat throughout the body while preserving skeletal muscle mass.
Leveraging the powerhouse of the cell to achieve better health
By harnessing the natural mechanism of mitochondrial uncoupling, Rivus’ therapies accelerate metabolism with the aim of enabling healthy lives for millions.
IMPROVE CELLULAR METABOLISM
Controlled metabolic accelerators (CMAs) like HU6 are oral, small molecule therapies designed to improve cellular metabolism. CMAs harness a natural metabolic process in mitochondria, the powerhouse of cells, to increase the breakdown of fats and sugars and increase resting energy expenditure.
REDUCE FAT, PRESERVE MUSCLE
By increasing energy expenditure, CMAs decrease fat throughout the body while maintaining skeletal muscle mass. Muscle retention is critical to minimize fat regain and reduce cardiometabolic risk.
CLINICAL
OPPORTUNITY
In a Phase 2 clinical trial, HU6, a first-in-class CMA, was shown to improve disease markers of blood pressure, inflammation and glycemic control. CMAs are now being studied to treat obesity and its comorbidities for patients suffering from heart failure, MASLD/MASH and type 2 diabetes.
OUR PIPELINE
CMAs have the potential to provide well-tolerated, effective treatments that improve cardiometabolic health with applicability to a broad range of diseases.
Clinical research demonstrates the potential of Rivus’ lead candidate HU6, a first-in-class CMA, to address a broad range of cardiometabolic diseases by increasing fat selective weight loss and improving key markers of glycemic control and inflammation. Importantly, no loss of lean muscle mass has been observed, suggesting reduced risk of weight regain and reduced cardiovascular risk.
For its lead program HU6, Rivus Pharmaceuticals has completed Phase 1 clinical studies and a Phase 2a metabolic study, which was published in The Lancet Gastroenterology & Hepatology. Building on data from the metabolic study, Rivus initiated a Phase 2a study in patients with obese heart failure with preserved ejection fraction (HFpEF) and a Phase 2b study in patients with metabolic dysfunction-associated steatohepatitis (MASH). Rivus is expanding its pipeline of CMAs targeting diseases where the evidence for safe metabolic acceleration is strong and the therapeutic need is great.
RIVUS CONTROLLED METABOLIC ACCELERATOR PIPELINE
Target Indication |
Candidate/Target |
Preclinical |
Phase I |
Phase II |
Phase III |
|---|---|---|---|---|---|
|
Obese HFpEF, MASH, T2D, Obesity
|
HU6
ANT-Activator |
||||
|
Obesity, CVD
|
RV-002
ANT-Activator |
ANT-activator
ANT-activator
HFpEF – heart failure with preserved ejection fraction; MASH – metabolic dysfunction-associated steatohepatitis; T2D – type 2 diabetes; CVD – cardiovascular disease; ANT – adenine nucleotide translocase
CLINICAL TRIALS
For more information on any Rivus-sponsored clinical trials, please contact our clinical operations team at info@rivuspharma.com.
Expanded Access PolicyEXPANDED ACCESS TO INVESTIGATIONAL MEDICINES
At this time, Rivus Pharmaceuticals does not provide access to investigational products outside of clinical trials. We encourage patients to participate in clinical trials of our investigational therapies whenever possible. Clinical trials are designed, conducted, and monitored to ensure that the safety and efficacy are appropriately evaluated before they are submitted to regulatory agencies for review with the intent to make them more broadly available to patients.
Our Team
The Rivus team is a group of forward-thinking scientists and proven biotech leaders deeply committed to the field of cardiometabolic health. Our team of experts is driven to provide patients and physicians with a new and highly effective solution to one of the most challenging issues in healthcare today.

CAREERS
Rivus has offices in San Francisco, California and Charlottesville, Virginia and is seeking candidates to join our growing team as we expand our pipeline of controlled metabolic accelerators (CMAs).
CONTACT
hr@rivuspharma.com
NEWS
Learn more about Rivus and controlled metabolic accelerators (CMAs).
PRESS RELEASES
Rivus Pharmaceuticals Announces Completion of Enrollment in Phase 2a HuMAIN Trial of HU6
Rivus Pharmaceuticals Announces Expansion of HU6 Clinical Program and New Leadership Appointments
Phase 2a Study of HU6, Rivus Pharmaceuticals’ Investigational Controlled Metabolic Accelerator, Demonstrates Clinical Benefit in Patients with High BMI and Nonalcoholic Fatty Liver Disease
Rivus Pharmaceuticals Announces Leadership Transition, Appointment of Jayson Dallas, M.D., as CEO
Rivus Pharmaceuticals to Present Results from a Phase 2a Trial Evaluating its Controlled Metabolic Accelerator, HU6, at the American Association for the Study of Liver Diseases (AASLD)
Rivus Pharmaceuticals Closes $132 Million Series B Financing to Advance HU6 for the Treatment of Obesity and Cardiometabolic Disorders
Rivus Pharmaceuticals Announces Positive Data from Phase 2a Clinical Trial of Lead Candidate HU6, Demonstrating Fat Reduction and Weight Loss in High BMI Participants
Rivus Pharmaceuticals Announces Positive Results from Phase 1 Trial of Lead Candidate HU6, Demonstrating Safety, Efficacy in Key Targets for Multiple Cardiometabolic Diseases
Rivus Pharmaceuticals Launches with $35 Million Series A Financing to Develop Novel Treatments for Cardiometabolic Diseases
NEWS
PRESS RELEASES
Rivus Pharmaceuticals Announces Positive Data from Phase 2a Clinical Trial of Lead Candidate HU6, Demonstrating Fat Reduction and Weight Loss in High BMI Participants
NEWS
PRESS RELEASES
Rivus Pharmaceuticals Announces Positive Data from Phase 2a Clinical Trial of Lead Candidate HU6, Demonstrating Fat Reduction and Weight Loss in High BMI Participants
- Met primary endpoint (liver fat reduction), multiple secondary endpoints (whole body, visceral, subcutaneous fat loss)
- Significant fat selective weight loss while preserving muscle mass, without changes in diet or exercise
- Amplified weight and fat loss in patients with elevated HbA1c
- Improvement in key markers of insulin resistance and inflammation
- Well tolerated across all studied doses
HU6 leads a pipeline of first-in-class, orally administered Controlled Metabolic Accelerators (CMAs) that accelerate fat metabolism and treat the underlying cause of type 2 diabetes, HFpEF, NASH, and other cardiovascular and metabolic diseases
CHARLOTTESVILLE, Va., February 9, 2022 – Rivus Pharmaceuticals Inc., a biopharmaceutical company dedicated to improving cardio-metabolic health, today announced positive results from a Phase 2a clinical trial of HU6 in obese participants with elevated liver fat. In eight weeks, HU6 demonstrated significant reductions in liver, visceral, and total body fat while conserving skeletal muscle mass, leading to significant reductions in total body weight. Uniquely, the greatest reductions in weight and body fat were observed in patients who had high baseline HbA1c levels. Improvements were also observed in key metabolic parameters that drive the pathophysiology of type 2 diabetes, heart failure with preserved ejection fraction (HFpEF), and non-alcoholic steatohepatitis (NASH). HU6 was well tolerated across all studied doses.
“We are highly encouraged by the Phase 2a clinical data evaluating HU6 to improve cardio-metabolic health. In this a relatively short study of 8 weeks, HU6 was able to achieve meaningful results safely in a high percentage of responders. By reducing fat in the liver, and throughout the body, HU6 addresses the root cause of poor metabolic health and related diseases,” said Allen Cunningham, President and CEO of Rivus Pharmaceuticals.
HU6 Phase 2a Metabolic Trial Design and Results
The phase 2a metabolic trial of HU6 was a 61-day randomized, double-blind, placebo-controlled trial designed to assess the safety and efficacy of three dose levels of HU6 (150 mg, 300mg, and 450 mg) in obese participants (body mass index 28 to 45 kg/m2) with elevated liver fat (greater than 8%). Eighty (80) participants between ages 28 and 65 were randomly assigned to one of three HU6 treatment groups or the matched placebo group, stratified and blocked for HbA1c levels of 5.7% or greater, and dosed once daily (fasting). Participants were instructed to not change behavior with regard to diet or exercise. The Phase 2a trial met primary (liver fat reduction by MRI-PDFF) and secondary (body weight and fat reduction by abdominal MRI) endpoints. Key results and observations include:
- Statistically significant (p<0.0001 by ANCOVA) reductions in liver fat at all three dose levels.
- Relative reductions in liver fat were 33%, 43%, and 40% corresponding to responder rates (>30% relative reduction) of 40%, 71% and 72% at low, mid and high doses, respectively, compared to placebo relative reduction in liver fat of 2% and responder rate of 5%.
- Body weight reduction almost exclusively from loss of fat, sparing skeletal muscle mass at all dosing levels at eight weeks, without change in diet or exercise behavior.
- Weight and fat loss showed a dose response with participants losing an average of 6 pounds (p<0.001, high dose vs. placebo).
- Participants with elevated HbA1c levels experienced greater weight and fat loss, losing an average of 10 pounds (p<0.0001, high dose vs. placebo).
- Fat loss was observed in hepatic, visceral, and subcutaneous compartments by MRI.
- Key cardiovascular and metabolic health indicators at all dosing levels including:
- Signficant dose dependent reduction in glycated albumin, an indicator of glucose control and insulin function.
- Significant dose dependent reduction in inflammation marker high sensitivity C-reactive protein (hsCRP), an important parameter of cardiovascular risk.
- HU6 was well-tolerated at all dose levels with excellent compliance. No Serious Adverse Events or deaths were reported. Intermittent diarrhea and transient flushing were the most commonly reported Treatment Emergent Adverse Events. The majority of these events were mild; one participant discontinued HU6 for diarrhea in the low dose arm while no participant discontinued at the high dose.
“This trial provides compelling proof of clinical concept for the efficacy and safety of mitochondrial uncoupling with HU6, our first in class CMA. HU6 pharmacology which selectively reduces fat and weight without appetite suppression is highly differentiated. Given early indications that this biology may reverse insulin resistance and systemic inflammation, we can now explore whether reducing visceral and organ fat can provide clinical benefits to patients across a host of cardiometabolic diseases,” said Shaharyar Khan, Ph.D., Rivus’ Chief Scientific Officer.
CMAs provide a new, measured approach to safely activating mitochondrial uncoupling, a process in the body that regulates and dissipates energy. By ferrying protons out of the mitochondrial intermembrane space, CMAs increase the oxidation of sugars and fats, while maintaining the same baseline production of adenosine triphosphate (ATP). Activating this process results in the reduction and the prevention of fat accumulation throughout the body.
In the first half of 2022, Rivus is continuing its HU6 clinical program with a Phase 2a study in heart failure with preserved ejection fracture (HFpEF) to be followed by Phase 2b studies in type 2 diabetes and non-alcoholic steatohepatitis (NASH).
About HU6
HU6 is a controlled metabolic accelerator (CMA) that provides a novel, measured approach to activating proton leak and mitochondrial uncoupling, a natural process in the body that regulates and dissipates energy. By ferrying protons out of the mitochondrial intermembrane space, CMAs cue the increased oxidation of sugars and fats, while maintaining the same baseline production of adenosine triphosphate (ATP). Activating this process results in the reduction of accumulated fat throughout the body.
About Rivus Pharmaceuticals
Rivus Pharmaceuticals, Inc., is dedicated to transforming the treatment of cardio-metabolic disease. Rivus’ first-in-class small molecule therapy, HU6, is a controlled metabolic accelerator (CMA) that addresses the underlying cause of cardio-metabolic disease by harnessing the body’s natural processes to improve cellular metabolism and selectively oxidize fat. Rivus’ therapy presents a tremendous opportunity to empower patients on their journey to better health when facing a broad range of cardio-metabolic conditions, including type 2 diabetes, heart failure with preserved ejection fraction (HFpEF), non-alcoholic steatohepatitis (NASH), and obesity. For more information, please visit rivuspharmadev.wpengine.com.
Rivus Pharmaceuticals Announces Positive Results from Phase 1 Trial of Lead Candidate HU6, Demonstrating Safety, Efficacy in Key Targets for Multiple Cardiometabolic Diseases
NEWS
PRESS RELEASES
Rivus Pharmaceuticals Announces Positive Results from Phase 1 Trial of Lead Candidate HU6, Demonstrating Safety, Efficacy in Key Targets for Multiple Cardiometabolic Diseases
HU6 was well tolerated and increased resting energy expenditure
Dose dependent weight loss and an improvement in metabolic parameters were also observed, despite the study not being powered to demonstrate them
Enrollment for a Phase 2a Metabolic Trial of HU6 has completed with topline data expected in Q1 2022
HU6 leads a pipeline of first-in-class Controlled Metabolic Accelerators (CMAs), designed to harness the body’s natural processes to improve cellular metabolism and treat underlying causes of poor metabolic health and cardiovascular disease
CHARLOTTESVILLE, Va. November 1, 2021 – Rivus Pharmaceuticals Inc., a biopharmaceutical company dedicated to improving cardio-metabolic health, today announced positive results from both single and multiple ascending dose portions of its Phase 1 clinical trial of HU6. HU6, a first-in-class, orally administered Controlled Metabolic Accelerator (CMA) therapy, demonstrated robust and sustained target engagement with no apparent safety signals. Additionally, sustained effects on key biological parameters relevant to cardio-metabolic diseases including type 2 diabetes, heart failure with preserved ejection fracture (HFpEF), non-alcoholic steatohepatitis (NASH), and severe hypertriglyceridemia (SHTG) were observed. HU6 was well-tolerated at all studied doses, and there were no serious adverse events. Participants dosed with HU6 demonstrated an increase in resting energy expenditure, dose dependent weight loss and improvements in metabolic parameters.
The HU6 Phase 1 program consisted of a double blind, placebo controlled, randomized single ascending dose (SAD) as well as a multiple ascending dose (MAD) trial. In the SAD portion of the program, 50 healthy volunteers received doses of HU6 ranging from 30 to 1400mg. In the MAD portion of the program, 24 subjects with high BMI (35-45) received doses of 200, 400, and 550 mg over 14 days of oral, once daily (QD) dosing. HU6 was well-tolerated at all dose levels. The limit to tolerability was not reached at any of the doses studied in the trial.
“We are highly encouraged by Phase 1 clinical trial results for HU6, which demonstrate a compelling, positive impact on multiple key indicators of cardio-metabolic disorders. The data overwhelmingly support the continued development of HU6 to deliver novel and effective treatment to the millions people who live with poor metabolic health and cardiovascular disease,” said Allen Cunningham, President and CEO of Rivus Pharmaceuticals. “While many existing cardio-metabolic therapies address downstream effects of these diseases, we’ve designed CMAs to address the underlying cause.”
CMAs provide a new, measured approach to activating proton leak and mitochondrial uncoupling, a natural process in the body that regulates and dissipates energy. By ferrying protons out of the mitochondrial intermembrane space, CMAs cue the increased oxidation of sugars and fats, while maintaining the same baseline production of adenosine triphosphate (ATP). Activating this process results in the reduction of accumulated fat and the prevention of additional fat accumulation throughout the body.
The company also announced it has completed enrollment for a double blind, placebo controlled, randomized Phase 2a clinical metabolic study of HU6 in cohorts of participants with high BMI (28-45 kg/m2). Topline data are expected in Q1 2022.
# # #
About Rivus Pharmaceuticals
Rivus Pharmaceuticals, Inc., is dedicated to transforming the treatment of cardio-metabolic disease. Rivus’ first-in-class small molecule therapy, HU6, is a controlled metabolic accelerator (CMA) that addresses the underlying cause of cardio-metabolic disease by harnessing the body’s natural processes to improve cellular metabolism. Rivus’ therapy presents a tremendous opportunity to empower patients on their journey to better health when facing a broad range of cardio-metabolic conditions, including type 2 diabetes, hypertension, non-alcoholic steatohepatitis (NASH), dyslipidemia and obesity. For more information, please visit rivuspharmadev.wpengine.com.
Rivus Pharmaceuticals Launches with $35 Million Series A Financing to Develop Novel Treatments for Cardiometabolic Diseases
NEWS
PRESS RELEASES
Rivus Pharmaceuticals Launches with $35 Million Series A Financing to Develop Novel Treatments for Cardiometabolic Diseases
Advancing a pipeline of first-in-class Controlled Metabolic Accelerators (CMAs) to harness the body’s natural processes to improve cellular metabolism and treat the underlying causes of poor metabolic health and cardiovascular disease
Initial programs to focus on type 2 diabetes, severe hypertriglyceridemia (SHTG), non-alcoholic steatohepatitis (NASH), heart failure with preserved ejection fraction (HFpEF)
CHARLOTTESVILLE, Va., July 20, 2021 /PRNewswire/ — Rivus Pharmaceuticals, Inc., a biopharmaceutical company dedicated to improving cardio-metabolic health, today announced the completion of a $35 million Series A financing. Rivus is advancing a new class of oral, once daily, small molecule therapeutics called Controlled Metabolic Accelerators (CMAs), designed to improve cellular metabolism and treat the underlying cause of highly-prevalent metabolic and cardiovascular diseases. The round was co-led by Longitude Capital and Medicxi and RxCapital also participated.
“More than 40 million people in the U.S. alone are living with cardio-metabolic disorders, conditions that adversely impact a person’s health and quality of life, as well as place significant cost burdens on healthcare systems. While many existing therapeutic options address the downstream effects of metabolic disease, they do little to address the underlying cause – greater consumption of energy than is required by the body, which over time results in inefficient cellular metabolism and the excess accumulation of fat,” said Allen Cunningham, President and CEO, Rivus Pharmaceuticals. “Our CMAs are designed to effectively target the root cause of these diseases by improving cellular metabolism with the potential to halt the progress of, or even reverse, these diseases.”
CMAs provide a new, measured approach to activating mitochondrial uncoupling, a natural process in the body that regulates and dissipates energy. By ferrying protons out of the mitochondrial intermembrane space, CMAs cue the increased oxidation of sugars and fats, while maintaining the same baseline production of adenosine triphosphate (ATP). Activating this process results in the reduction of accumulated fat and the prevention of additional fat accumulation throughout the body. Rivus is currently conducting a Phase 2a clinical study with its lead CMA therapeutic, HU6. The Series A financing will enable Rivus to advance a pipeline of CMA therapies to treat a range of metabolic conditions including type 2 diabetes, severe hypertriglyceridemia (SHTG), non-alcoholic steatohepatitis (NASH), as well as cardiovascular diseases such as heart failure with preserved ejection fraction (HFpEF).
“We are very encouraged by early clinical results of HU6 and the potential for CMAs to deliver a new and highly-effective approach to improving metabolic health,” said Patrick Enright, Managing Director at Longitude Capital. “There is tremendous global potential for CMAs to fundamentally change the therapeutic landscape across a variety of difficult to manage chronic diseases related to metabolic health.”
“Rivus has recently completed its Phase 1 program for HU6, and the results exceeded our expectations, demonstrating an early positive impact on key metabolic parameters, while being well-tolerated,” said David Grainger, Ph.D., Chief Scientific Advisor at Medicxi. “Phase 2 studies will provide important insights into HU6’s efficacy in specific metabolic and cardiovascular diseases with this very promising new therapy, and we are excited to advance these programs in partnership with Rivus.”
# # #
About Rivus Pharmaceuticals
Rivus Pharmaceuticals, Inc., is dedicated to transforming the treatment of cardio-metabolic disease. Rivus’ first-in-class, small molecule therapy, HU6, is a controlled metabolic accelerator (CMA) that addresses the underlying cause of cardio-metabolic disease by harnessing the body’s natural processes to improve cellular metabolism. Rivus’ therapy presents a tremendous opportunity to empower patients on their journey to better health when facing a broad range of cardio-metabolic conditions, including type 2 diabetes, hypertension, non-alcoholic steatohepatitis (NASH), dyslipidemia and obesity. For more information, please visit rivuspharmadev.wpengine.com.
About Longitude Capital
Longitude Capital is a leading healthcare venture capital firm that invests in transformative biotechnology, medical technology and health solutions companies seeking to improve clinical outcomes, enhance quality of life, and drive efficiency of healthcare delivery. Founded in 2006, Longitude Capital invests in both privately held and publicly traded companies through a variety of investment approaches. Longitude Capital has offices in Menlo Park, CA, Greenwich, CT, and Boston, MA. For more information, please visit www.longitudecapital.com or LinkedIn.
About Medicxi
Medicxi is an international investment firm with the mission to create and invest in companies across the full healthcare continuum. Medicxi was established by the former Index Ventures life sciences team and invests in both early stage and late stage therapeutics with a product vision that can fulfill a clear unmet medical need. GlaxoSmithKline, Johnson & Johnson Innovation – JJDC, Inc., Novartis and Verily (an Alphabet company) are investors in Medicxi funds.
Medicxi’s team has been investing in life sciences for over 20 years. Globally, it has invested in 91 innovative biopharma companies and achieved 32 exits through IPO and M&A, including Genmab, PanGenetics (sold to AbbVie), Cellzome (sold to GSK), Micromet (sold to Amgen), Molecular Partners, XO1 (sold to Janssen Pharmaceuticals, Inc.), Minerva Neurosciences, Padlock Therapeutics (sold to Bristol-Myers Squibb), Gadeta (structured transaction with Gilead), Impact BioMedicxines (sold to Celgene) and Adaptive Biotechnologies. Medicxi is also the founding investor of Centessa Pharmaceuticals. Please see https://www.medicxi.com/ for more information.
About RxCapital
RxCapital is the venture capital arm of RxCelerate, a leading international out-sourced drug discovery platform based in Cambridge, UK. RxCapital invests in early-stage biotechnology companies with transformative capability and, unlike many CROs, there is no requirement for portfolio companies to use RxCelerate services. RxCapital will invest in any geography, following an asset-centric investment model, deploying up to £1million per investment. For further information, please contact Nick Tait, Chief Financial Officer of RxCelerate Group: nick@rxcelerate.com.
MEDIA CONTACT
info@rivuspharma.com